We Utilize Emerging Technologies to Study and Improve Muscle Regeneration.
We focus on the study of Muscle Stem Cell Biology in the fields of Development and Disease.
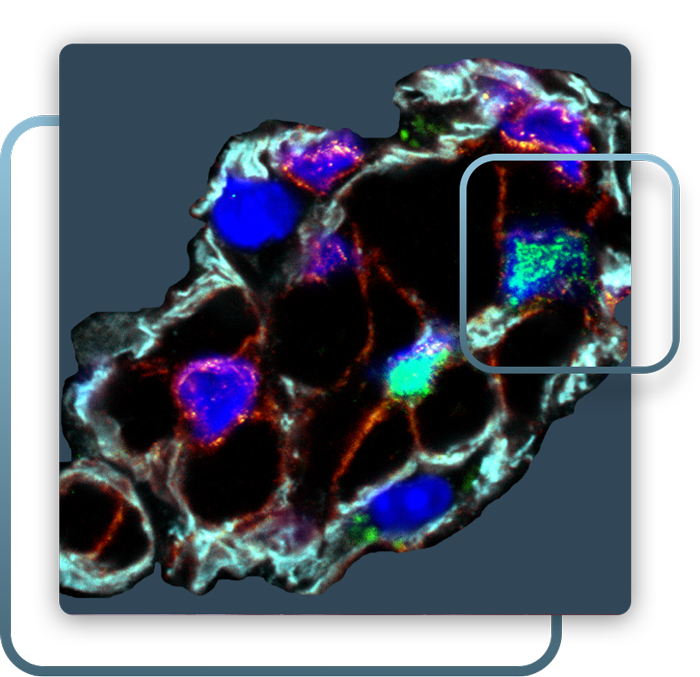
PLURIPOTENT STEM CELLS
Human pluripotent stem cells (hPSCs) offer a powerful model to study human development in vivo. HPSCs expand indefinitely and can be genetically modified in manners that improve engraftment potential, through modulating cell competition, survival, and niche formation. Recapitulating the complexity of skeletal myogenesis in vitro from hPSCs has presented numerous challenges for the field and my lab seeks to continue to improve directed differentiation of hPSCs to skeletal muscle.

"Skeletal muscle is one of the most regenerative tissues in the body due to the endogenous muscle stem cells called satellite cells."
STEM CELL NICHE
The stem cells of highly regenerative tissues associate with and are located in specialize compartments termed niches. Muscle stem cell niches are comprised of the myofiber sarcolemma and a laminin rich basal lamina containing dynamic ligand-receptor parts that support the stem cell. My work seeks to identify how emerging niches form and provide a new model for understanding human stem cell niche formation. Evaluating the regulators of emerging human niche formation during regeneration and development will improve our ability to generate de novo human niches and better support human muscle stem cells in vivo for cell therapy.

"Supporting skeletal muscle stem cells through interactions with the stem cell niche and microenvironment in development and disease."
STEM CELL TECHNOLOGY
My lab seeks to apply technology advancements in single cell biology, lineage tracing, imaging, and molecular biology to improve myogenesis from skeletal muscle stem cells.
- Single cell transcriptome and proteoics to study cell fate transitions
- CRISPR / Cas9 engineering in hPSCs
- Intravital microscopy to image in vivo stem cell behavior
- Transgenic mouse models to study the microenvironment effects on stem cells
- Bioengineering and co-culturing to reconstruct the niche to support muscle stem cells ex vivo
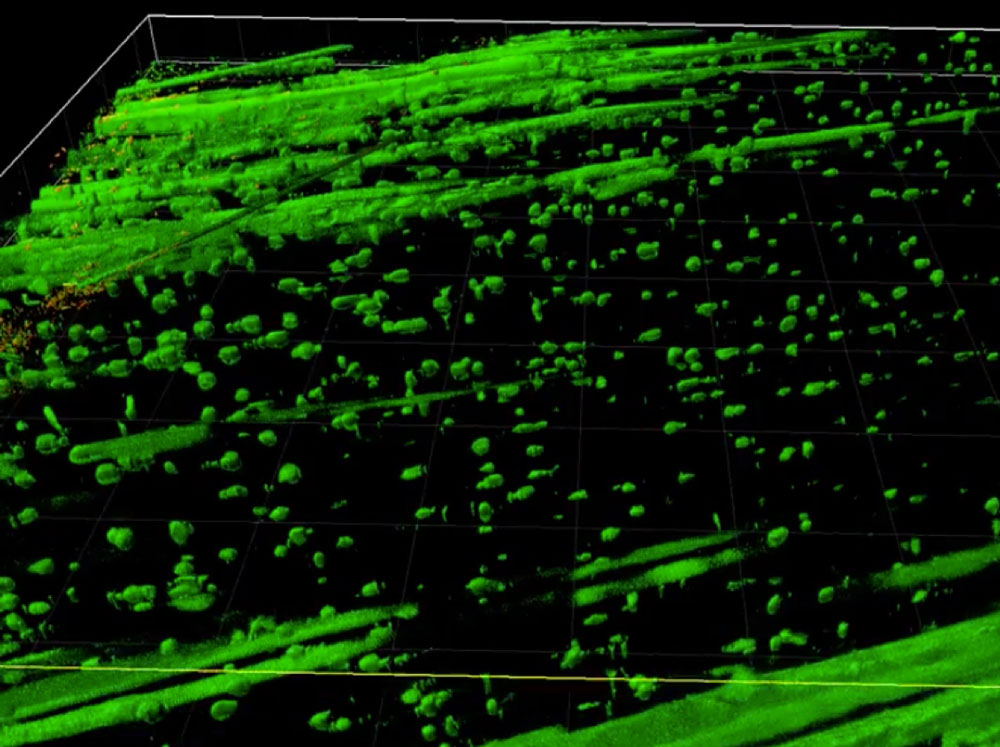

"We are using emerging technologies including lineage tracing, single-cell biology, and molecular engineering to improve our understanding of muscle stem cells and cell transplantation."
NEUROMUSCULAR DISEASE
In muscle wasting diseases such as DMD, the endogenous satellite cells become exhausted over time and get replaced by fat and fibrotic tissue. The ability to generate an efficient protocol for producing skeletal muscle from PSCs provides a unique in vitro model for improving our understanding of the consequences of dystrophin loss in human skeletal muscle.
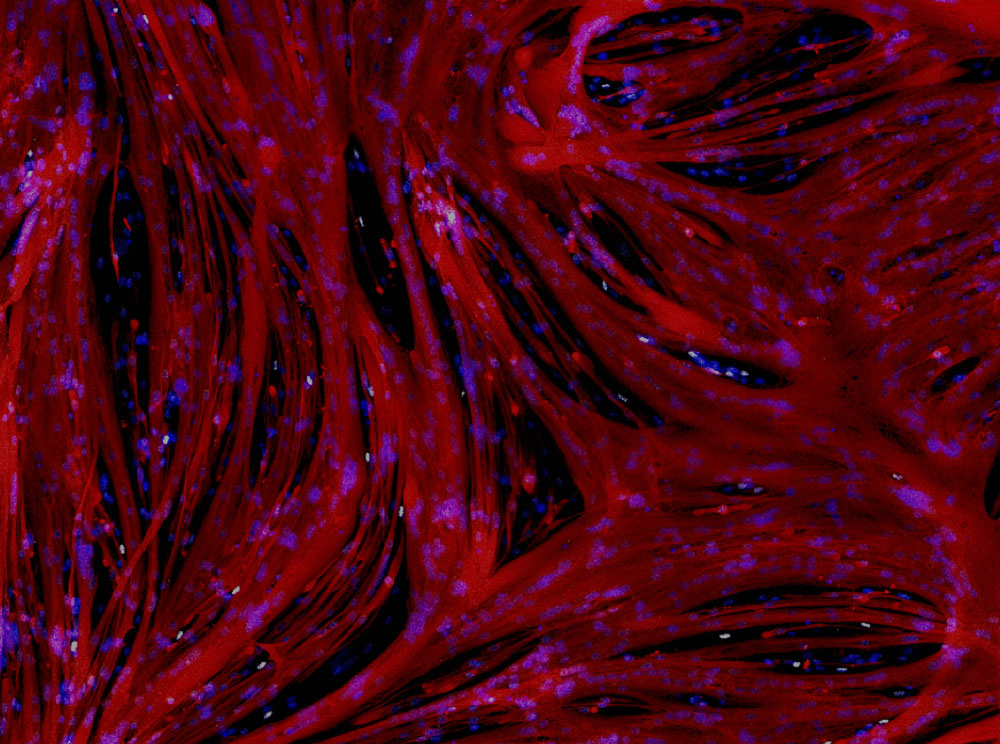

"“We are particularly interested in the microenvironment and how stem cell niches form and can be used to Improve personalized cell+gene therapies for neuromuscular diseases.” "
CELL THERAPY
Autologous or immune-tolerant allogeneic donor muscle cells can be used to repair or replace skeletal muscle wasting and improve functional outcomes for disorders ranging from cancer cachexia to age-related muscle loss.


""The Hicks lab is focused on overcoming four key barriers to stem cell therapy for muscle disease. These include 1. Increasing stem cell potency by creating muscle cells in the dish that behave like muscle cells in the body. 2. Supporting muscle engraftment to improve long-term functional outcomes. 3. Selecting appropriate human disease indications and developing preclinical models of disease, and 4. Understanding tolerance and safety for human use.""
Featured Videos
Inspiration for UCI Anti-Cancer Challenge
VanAnh’s 3d Bioprinting
IN THE NEWS
NEWS AND NOTEWORTHY
)
ABOUT DR. MICHAEL HICKS
Dr. Michael Hicks joined UC Irvine and the Stem Cell Research Center as an assistant professor in 2020. Dr. Hicks received his PhD in 2014 with Dr. Paul Standley at the University of Arizona and Arizona State University, studying how connective tissue fibroblasts and mechanical strain influenced skeletal muscle regeneration. Dr. Hicks did his postdoctoral research at UCLA with Dr. April Pyle where he was the first to define the developmental and functional identify of skeletal muscle cells generated from human pluripotent stem cells (hPSCs).

JOIN THE TEAM
Looking for an exciting career in a quickly evolving field of research? Contact us today for more information about our research or working with Hicks Laboratory.
VIEW AVAILABLE POSITIONS)
Olga G. Jaime
PhD Student
Training: Cal State Long Beach, UC Irvine
Research: Stem cell fate decisions, ERBB3/EGFR signaling, Neuromuscular formation
)
Ben Clock
PhD Student
Training: Cal Poly Pomona, Salk Institute
Research: Stem cell niche formation, CRISPR/Cas9 engineering, MuscleMatchR
)
Amanda Tedesco
Junior Specialist, Medical Student
Training: UC Riverside, UCI Medical School
Focus: Orthopedic Surgery, Rotator Cuff Repair
)
Manuel Michel
Master’s Student
Training: CalPoly Pomona
Focus: Visiopharm AI Deep Learning, Fibro/adipogenic progenitors
)
Shanice Benson
Master’s Student
Training: Maastricht University
Focus: Limb organoids, Lineage commitment
)
Shreya Pavani
Senior Research Associate 1
Training: UC Irvine, Neurobiology
Focus: Lab Management, Neuromuscular Disorders, Gene Editing
)
Joanne Kim
Lab Technician
Training: UC Irvine, Genetics
Focus: Rotator Cuff Pathology
)
Sebastien Colobong
Student Researcher
Training: UC Irvine, Biology
Focus: SC Niches, Plasmid Design
)
Vananh Nguyen
Student Researcher
Training: UC Irvine, Biology
Focus: 3D Bioprinting, Organoids
)
Nikitha Pavar
Student Researcher
Training: UC Irvine, Biology
Focus: Stem cell niche
)
Tyler Huang
Student Researcher
Training: UC Irvine, Biology
Focus: Clinical Muscle Pathology and Imaris Analysis
Alumni
2021-2022
Current position: Nutcracker Therapeutics in San Francisco, CA
2023-2024
Current position: UCI medical student in Irvine, CA